Researchers overcome stem cell delivery barrier, paving the way for regenerative medicine

DNA being delivered into the nucleus of a stem cell - An artistic rendition of the study led by Dr Gang Ruan and selected as a supplementary cover art by the journal Nano Letters. Credit: American Chemical Society (Nano Letters)
A recent study has introduced a new method for delivering particles into stem cells, which are notoriously difficult to penetrate. The discovery will make it easier to direct and enhance the processes involved in regenerative medicine.
Regenerative medicine takes advantage of the fact that our body's stem cells can change into many other cell types that are vital for the regeneration of tissue and organs, such as heart or nerve cells.
Each type of cell has specialised properties and functions, so harnessing the potential of stem cell development means that regenerative medicine offers some of the most promising treatments for many diseases.
To control the type of cell the stem cells change into, scientists need to reprogram the cells' genes by inserting genetic information into the stem cell's nucleus, as an operator would adjust railway tracks to change the direction of a train.
However, stem cells have robust protection to stop anything from getting in, similar to our skin, so manipulating the differentiation of stem cells has been problematic.
The researchers have been working to overcome this using rat stem cells and have created a way to bypass the cells' protective barrier.
Dr Gang Ruan, from Xi'an Jiaotong-Liverpool University (XJTLU), China, and a corresponding author, says: "Our new method means we can deliver genetic information to the stem cells more quickly and efficiently and control the kind of cell they become."
Dr Xiaowei Wen, also from XJTLU and a corresponding author, adds: "Being able to control cell differentiation with this new method means we can improve the efficiency of stem cell therapy as we can better control what the cells transform into. This means fewer cells will be wasted, and we will need fewer cells overall to help regenerate or repair damaged tissue and organs.
"This, in turn, brings down the cost and increases the quality of the patient's life as stem cells can be used rather than donor organs which have a limited supply."
Scaffolding solutions
Dr Wen explains why the new technology is needed to take advantage of the unique properties of stem cells.
"As we age, the number of stem cells in our body decreases dramatically. So, to harness their potential to regenerate damaged cell tissue and organs, we need to implant them into the body.
"Unfortunately, the introduced stem cells usually die within about a week once they are in the body, yet can take around four weeks to differentiate into other cell types.
"So, in our lab, we grow stem cells outside the body. Then using our new method, we can insert specific genetic information into the cells using nanoparticles to cause them to change into a particular type of cell.
"Once the cells have differentiated into the target cell type, we put them into the area of the body where there is damaged tissue so that they can help to restore it."
In a previous study, the team identified the bottleneck in the process of delivery of nanoparticles to stem cells. They showed that the nanoparticles got trapped in bubble-like vesicles that prevented them from getting into the stem cell, but it wasn't clear why.
Breached barriers
To understand how to overcome the difficulties posed by the stem cell barrier, the team of researchers studied ways to improve the movement of nanoparticles across the cell membranes, which could carry genetic information that would direct the transformation of a stem cell to its new cell type.
"We tried many methods that have worked in other cell types, and we found that most of these failed miserably, even ones we had high hopes for," Dr Ruan says.
"Eventually, we found that coating the nanoparticles in a type of polymer helped them to get into the stem cells.
"The coated nanoparticles avoided getting trapped in vesicles, unlike the uncoated ones. In fact, they seemed to circumvent the vesicles altogether and enter the cell more directly.
"It actually wasn't a method we expected to be successful."
It's not yet clear why the coating works, but the discovery will help to make the delivery of genetic information to stem cells more efficient so that it is easier to control which cells they become.
However, the team recognises there is a long way to go before this method can be used clinically.
Dr Ruan says: "Not only do we need to further optimise delivery into the cells, but we also need to precisely control when it happens."
"But it's a big step in the right direction."
Discovering through destruction
Although the discovery that it was easier for coated nanoparticles to pass into stem cells has helped to solve the delivery problem, the fundamental question of why stem cells are so difficult to enter remains.
The team, therefore, looked at the barrier surrounding the stem cells, the cell membrane, to see which characteristics gave them such unique properties.
They took stem cell samples from six rats and used a device called a sonicator, like a mini pneumatic drill, to break up the cells, then measured the amount of damage.
They found that stem cell membranes were more difficult to break when compared to other cell types that are easier to transfer genetic information into.
"Stem cell membranes seemed more robust than other cell types when sonicated. The preliminary results of the study also show that the stem cells contain more cholesterol in their cell membranes," says Dr Ruan.
"This extra cholesterol makes the membrane more rigid, similar to the problems caused by cholesterol in our blood vessels. This may be why it is so difficult for nanoparticles to pass through the membrane of stem cells, though much more research is required to confirm this."
Although the results are preliminary, this understanding of stem cell properties will further aid the development of stem cell delivery using coated nanoparticles and the optimisation of future regenerative therapies.
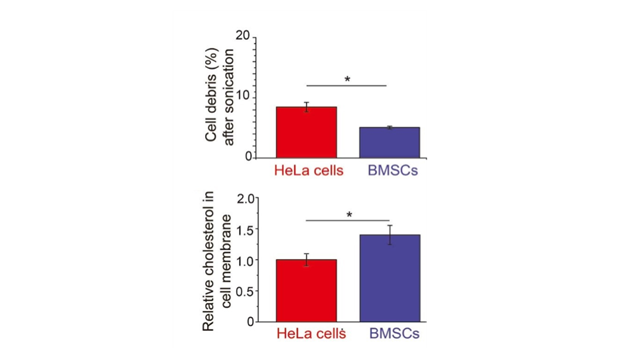
Bone marrow-derived mesenchymal stem cells (BMSCs) were less damaged after sonication than other cell types (top), measured by flow cytometry. BMSCs also had more cholesterol in their cell membranes (bottom). This could be why it is more difficult to penetrate stem cells
The study, Mechanism-driven technology development for solving the intracellular delivery problem of hard-to-transfect cells, can be read here.